North America Clinical Trials Market- Industry Analysis and Forecast (2025-2032)
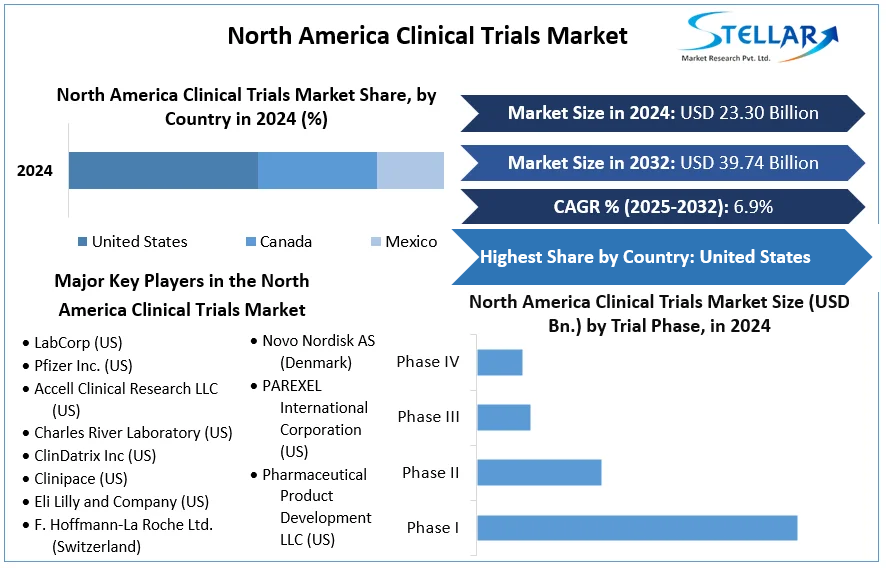
North America Clinical Trials Market size was valued at US $ 23.30 Billion in 2024 and the total Market revenue is expected to grow at 6.9% through 2025 to 2032, reaching nearly US $ 39.74 Billion.
Format : PDF | Report ID : SMR_821
North America Clinical Trials Market Overview:
Clinical Trials Market report examines the market’s growth drivers as well as its segments (Application, Technology and Region). This market study takes an in-depth look at all of the significant advancements that are currently occurring across all industry sectors. To provide key data analysis for the historical period (2019-2024), statistics, and presentations are used. The report examines the Clinical Trials Market Drivers, Restraints, Opportunities, and Challenges. This SMR report includes investor recommendations based on a detailed analysis of the current competitive landscape of the Clinical Trials Market.
The North America Region leads the world in the Clinical Trials Market. This can be attributed to countries such as the United States of America and Canada, which are home to many key players in the Pharmaceutical and Biotechnology markets. According to the United States National Library of Medicine, 32% of all clinical trials held globally were located only in the United States of America. The Food and Drug Administration of the United States has become one of the world's leading institutions for conducting and analysing controlled clinical drug trials. Various legislation passed by the U.S. government throughout the ’60s mandated the FDA’s approval of drugs based on scientific research and substantial evidence of a drug’s efficacy, paving the way for intensive clinical trials leading to significant growth of the Clinical Trials Market in North America.
Today the region is a pioneer in modern ways of conducting clinical trials as well as using advanced Bioanalytic for the statistical study of the results. The Clinical Trials Market in the region is driven by the growing number of medical trials being conducted, the increasing demand for novel therapies, focus on orphan and rare diseases, gene therapy treatments, and an increase in the incidence of diseases such as diabetes and cancer in the population, and the outbreak of diseases such as COVID 19.
The demand for clinical trials is further boosted by the incentives provided by the government and the investments made by the key players in Research and Development. The region is also home to many Contract Research Organisations(CROs) that work on research data for clinical trials on a contract basis. All these factors signify a sustained demand for clinical trials in the forecast period and therefore promise substantial growth of the Clinical Trials Market in the North America Region.
To get more Insights: Request Free Sample Report
North America Clinical Trials Market Dynamics:
The increased research for novel drugs and the increasing incidence of infectious diseases drive the Clinical Trials Market in the North America Region. The region is home to many pharmaceutical giants that pioneer in drug manufacturing and research. As a result, these companies conduct a significant number of clinical trials for various diseases
The population of the region exhibits an increased marker for diseases, which leads to more research and therefore increase in the number of clinical trials in the region. The significant geriatric population in countries such as the US and Canada is susceptible to a number of diseases and ailments that come with ageing. The region is therefore invested in finding treatments and drugs to overcome the diseases which would require significant drug testing before being used by the populations. The region also hosts a significant Cosmetic Surgery market, consumers of which use the procedure for correcting birth defects, changing their appearance, and reversing the effects of ageing. The market requires significant clinical trials before the development of new procedures, which leads to the growth of the Clinical Trials Market in the region.
The region hosts significant research in Gene Therapy, which is the replacement of defective or missing genes with normal genes to correct genetic disorders. The promising applications of the treatment method, specifically in the treatment of orphan and rare diseases promise significant research and study at both medical and institutional levels through clinical trials, leading to the growth of the Clinical Trials Market in the North America Region.
As a result of the region being host to leaders in wearable technology that provide accurate vital monitoring, the region is changing the way in which research data is collected in medical trials. Modern wearable consumer electronics have a variety of advanced, dependable, and accurate sensors that medical personnel can utilize to remotely monitor their patients. The technology is also being utilized in decentralized medical studies, where researchers track changes in vital signs over time in response to a lifestyle change or pharmacological use. Wearables enable continuous monitoring of patients who are no longer constrained by geography, which has resulted in substantial changes to medical Clinical Trials methodology.
North America Clinical Trials Market Segment Analysis:
Based on the Trial Phase, the Clinical Trials Market for the North America region is segmented into Phase I, Phase II, Phase III and Phase IV segments. The Phase III Segment dominated the Clinical Trials Market in the year 2024. According to the National Clinical Trials Registry(NCT), there are roughly 309 Cardiovascular disease Phase 3 trials being conducted in the region. The Phase I segment is predicted to grow the most during the forecast period. This is attributed to the growing Investigative New Drugs(IND) market for the treatment of orphan and rare diseases that are especially being researched in the region. Phase III trials evaluate the effectiveness and safety of a new drug being introduced to the market. As a result, the studies that need to be conducted require a large number of volunteers to study and are therefore large scale, requiring significant capital. As a result, the Phase III segment dominates the market and is forecasted to continue the trend during the study period.
Based on Service Type, the Clinical Trials Market for North America is segmented into Protocol Designing, Site Identification, Patient Recruitment, Laboratory Services, Bioanalytical Testing, Analytical Testing, Clinical Trial Supply & Logistic Services, Decentralized Clinical Services, Clinical Trial Data Management Services, Medical Device Testing Services, and other services. The Clinical Trial Data Management segment dominated the market in the year 2024. This is because Clinical Trial Data Management is a crucial stage in clinical research that results in high-quality, reliable, and statistically sound data from clinical trials. This contributes to a significant reduction in the period between drug development and marketing. This is imperative for the profit models of players and investors in Drug Companies as delays in the phase lead to huge losses. Patient Recruitment and Analytical Testing segments for the North America Region are also expected to grow significantly during the forecast period.
Based on the Therapy Area, the Clinical Trials Market for North America is segmented into Oncology, Infectious Diseases, Cardiology, Neurology, Women’s Health, Genetic Diseases, Immunology, and Others. The Oncology segment dominated the North America Clinical Trials Market in 2024. Other segments that held significant market share were Cardiology, Neurology and Infectious Diseases. This can be attributed to the widespread incidence of diseases such as obesity and diabetes in the population of countries such as the United States and Canada. The Infectious Diseases segment in the North America Region saw drastic growth during the Covid 19 Pandemic. This was because the unprecedented rapidity of the development of the Covid Vaccine saw a coordinated effort by vaccine manufacturers, governments and regulatory bodies. Companies such as Pfizer, Moderna and others performed fast-tracked clinical trials in modern ways leading to significant growth of the Clinical Trials Market in the North America Region.
The report provides Porter’s Five Forces Model that will aid enterprise decision makers to develop their long-term strategies and marketing positions. It identifies the key competitors, their positioning and how their products perform and are perceived by the customers. Through expert analysts’ opinion, the report provides consultation on the difficulty of entering the market for new players.
Further the report provides PESTEL Analysis which will help the company tailor the overall strategies for market activities. The analysis takes into consideration various environmental variables that are crucial for the development of the market position of the company such as government funding and other political variables. Therefore, this analysis helps the stakeholder evaluate the challenges and opportunities in a holistic way.
North America Clinical Trials Market Scope:
|
North America Clinical Trials Market |
|
|
Market Size in 2024 |
USD 23.30 Bn. |
|
Market Size in 2032 |
USD 39.74 Bn. |
|
CAGR (2025-2032) |
6.9% |
|
Historic Data |
2019-2024 |
|
Base Year |
2024 |
|
Forecast Period |
2025-2032 |
|
Segment Scope |
By Trial Phase
|
|
By Service Type
|
|
|
By Therapy Area
|
|
|
Country Scope |
United States Canada Mexico |
North America Clinical Trials Market Key Players:
- LabCorp (US)
- Pfizer Inc. (US)
- Accell Clinical Research LLC (US)
- Charles River Laboratory (US)
- ClinDatrix Inc (US)
- Clinipace (US)
- Eli Lilly and Company (US)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- ICON PLC (Ireland)
- IQVIA Holdings, Inc. (US)
- Novo Nordisk AS (Denmark)
- PAREXEL International Corporation (US)
- Pharmaceutical Product Development LLC (US)
- PRA Health Sciences (US)
- Sanofi SA (France)
- SGS SA (France)
- Syneos Health Inc. (US)
- Wuxi AppTec Inc. (China)
Frequently Asked Questions
The United States of America contributes the most to the Clinical Trials Market in the North America Region.
The key players in the Clinical Trials Market in the North America Region are Pfizer and LabCorp from the United States of America.
1. North America Clinical Trials Market: Research Methodology
1.1 Research Data
1.1.1. Primary Data
1.1.2. Secondary Data
1.2. Market Size Estimation
1.2.1. Bottom-Up Approach
1.2.2. Top-Down Approach
1.3. Market Breakdown and Data Triangulation
1.4. Research Assumption
2. North America Clinical Trials Market Executive Summary
2.1. Market Overview
2.2. Market Size (2024) and Forecast (2025 – 2032) and Y-O-Y%
2.3. Market Size (USD) and Market Share (%) – By Segments
3. North America Clinical Trials Market: Competitive Landscape
3.1. SMR Competition Matrix
3.2. Key Players Benchmarking
3.2.1. Company Name
3.2.2. Headquarter
3.2.3. Business Segment
3.2.4. End-user Segment
3.2.5. Y-O-Y%
3.2.6. Revenue (2024)
3.2.7. Profit Margin
3.2.8. Market Share
3.2.9. Company Locations
3.3. Market Structure
3.3.1. Market Leaders
3.3.2. Market Followers
3.3.3. Emerging Players
3.4. Consolidation of the Market
3.4.1. Strategic Initiatives and Developments
3.4.2. Mergers and Acquisitions
3.4.3. Collaborations and Partnerships
4. North America Clinical Trials Market: Dynamics
4.1. North America Clinical Trials Market Trends
4.2. North America Clinical Trials Market Drivers
4.3. North America Clinical Trials Market Restraints
4.4. North America Clinical Trials Market Opportunities
4.5. North America Clinical Trials Market Challenges
4.6. PORTER’s Five Forces Analysis
4.6.1. Intensity of the Rivalry
4.6.2. Threat of New Entrants
4.6.3. Bargaining Power of Suppliers
4.6.4. Bargaining Power of Buyers
4.6.5. Threat of Substitutes
4.7. PESTLE Analysis
4.7.1. Political Factors
4.7.2. Economic Factors
4.7.3. Social Factors
4.7.4. Technological Factors
4.7.5. Legal Factors
4.7.6. Environmental Factors
4.8. Technological Roadmap
4.9. Regulatory Landscape
5. North America Clinical Trials Market: Market Size and Forecast by Segmentation (by Value in USD Million) (2024-2032)
5.1. North America Clinical Trials Market Size and Forecast, by Trial Phase (2024-2032)
5.1.1. Phase I
5.1.2. Phase II
5.1.3. Phase III
5.1.4. Phase IV
5.2. North America Clinical Trials Market Size and Forecast, by Service Type (2024-2032)
5.2.1. Protocol Designing
5.2.2. Site Identification
5.2.3. Patient Recruitment
5.2.4. Laboratory Services
5.2.5. Bioanalytical Testing
5.2.6. Analytical Testing
5.2.7. Clinical Trial Supply & Logistics Services
5.2.8. Decentralized Clinical Services
5.2.9. Clinical Trial Data Management Services
5.2.10. Medical Device Testing Services
5.2.11. Other Services
5.3. North America Clinical Trials Market Size and Forecast, by Therapy Area (2024-2032)
5.3.1. Oncology
5.3.2. Infectious Disease
5.3.3. Cardiology
5.3.4. Neurology
5.3.5. Women’s Health
5.3.6. Genetic Disease
5.3.7. Immunology
5.3.8. Others
5.4. North America Clinical Trials Market Size and Forecast, by Country (2024-2032)
5.4.1. United States
5.4.2. Canada
5.4.3. Mexico
6. Company Profile: Key Players
6.1. LabCorp
6.1.1. Company Overview
6.1.2. Business Segment
6.1.3. Financial Overview
6.1.3.1. Total Revenue
6.1.3.2. Segment Revenue
6.1.4. SWOT Analysis
6.1.5. Strategic Analysis
6.1.6. Recent Developments
6.2. Pfizer Inc.
6.3. Accell Clinical Research LLC
6.4. Charles River Laboratory
6.5. ClinDatrix Inc
6.6. Clinipace
6.7. Eli Lilly and Company
6.8. F. Hoffmann-La Roche Ltd
6.9. ICON PLC
6.10. IQVIA Holdings, Inc.
6.11. Novo Nordisk AS
6.12. PAREXEL International Corporation
6.13. Pharmaceutical Product Development LLC
6.14. PRA Health Sciences
6.15. Sanofi SA
6.16. SGS SA
6.17. Syneos Health Inc.
6.18. Wuxi AppTec Inc.
7. Key Findings
8. Industry Recommendations
8.1. Strategic Recommendations
8.2. Future Outlook
















