North America Influenza Diagnostic Market: Size, Share, Trends- 2032
The North America Influenza Diagnostic Market was estimated at USD 575 Mn. in 2024 and is expected to grow at a CAGR of 6.7% from 2025 to 2032, reaching nearly USD 966.01 Mn. by 2032.
Format : PDF | Report ID : SMR_2880
Influenza Diagnostic Market refers to the global or regional industry segment focused on the development, production, distribution, and use of tests and technologies designed to detect influenza viruses (such as Influenza A, Influenza B, and their subtypes) in human or animal samples. Government initiatives by agencies such as the CDC (Centers for Disease Control and Prevention) It promote large-scale influenza surveillance, further fueling market demand. Post-pandemic focus on detection of respiratory virus has also increased multiplex molecular testing platforms. Diagnostic test volumes in the region are sufficient, with more than 200 million influenza tests alone in 2024. Of these, about 70% was rapid influenza diagnostic test. The United States is a dominating country, holding about 83% of regional revenue, due to robust diagnostic infrastructure, advanced laboratory and widespread insurance coverage. With continued importance on early detection, precision diagnostics, and epidemiological surveillance, the North America influenza diagnostics market is expected to maintain its lead over the forecast period (2025–2032).

To get more Insights: Request Free Sample Report
North America Influenza Diagnostics Market Dynamics
Increasing Global Incidence of Influenza Outbreaks to Boost the Influenza Diagnostic Market
According to World Health Organization, influenza results in 3 to 5 million serious diseases and 650,000 respiratory deaths every year. It has a bearing on an Increase in versions being tested during flu seasons, both laboratory-based and point-of-care testing solutions. advances in molecular diagnostics, like RT-PCR and isothermal nucleic acid amplification have greatly improved test sensitivity and specificity, driving their adoption both in clinical and point-of-care settings. The increasing rates and unpredictability of influenza viruses continue to be the most significant drivers in the North America influenza diagnostics market.
Market Faces Restraints Related to Limited Accessibility of Advanced Diagnostic Technologies
A major restraint for the North America influenza diagnostics market is that the most accurate tests, like RT-PCR, are expensive and not easy to access especially in smaller clinics or lower-income countries. These tests cost USD 50 to USD 150 and need special machines and trained staff. rapid antigen test are much cheaper but aren't always reliable, with accuracy sometimes as low as 50–70%. The RT-PCR test unavailable in rural hospitals, small clinics, or in many low-income countries. For example, while urban hospitals may have full molecular labs, millions of people living in rural areas still depend on basic rapid tests even they are less accurate.
Home-Based Influenza Testing to create Opportunities for Influenza Diagnostic Market
A rapidly emerging opportunity in the North America influenza diagnostics market is the rise of home-based flu testing, driven by telehealth integration and smart diagnostic devices. This trend is opening doors for app-connected test kits, AI-powered symptom checkers, and remote diagnosis services. Tech-forward diagnostics companies are developing FDA-authorized home-use flu test kits, this test kits deliver results in under 30 minutes. Some even connect directly to mobile apps, allowing patients to consult doctors virtually and get prescriptions in real-time.
North America Influenza Diagnostics Market Segment Analysis
Based on Product, the North America influenza diagnostics market is segmented into test kits and reagents, Instruments, and other products. The test kits and reagents segment dominated the market in 2024 and is expected to hold the largest market share over the forecast period (2025-2032). This dominance is driven by the high volume and repeat usage of diagnostic kits across clinical, laboratory, and at-home settings. It covers 62% of revenue in 2024, test kits are consumables required for each patient sample, leading to consistent demand. The segment includes rapid antigen kits for point-of-care use, RT-PCR and reagents for ELISA. In addition, the market access has been expanded for these products by increasing the home use kit and the government funded flu monitoring programs.

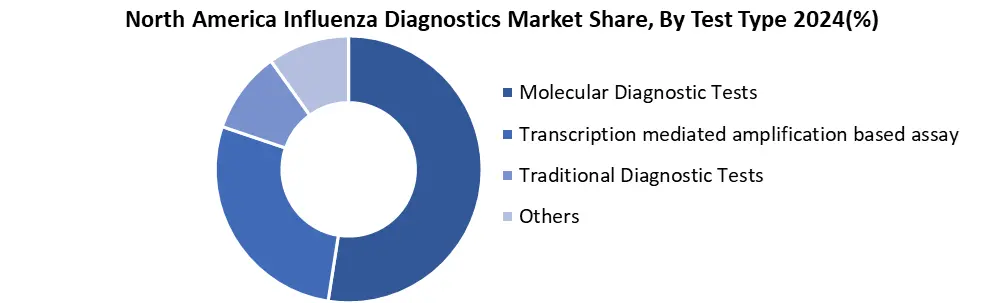
Based on Test Type, the North America influenza diagnostics market is segmented into Molecular Diagnostic Tests, Transcription-Mediated Amplification-Based Assay, Traditional Diagnostic Tests and others. The molecular diagnostic segment dominated the market in 2024 and is expected to hold the largest market share over the forecast period (2025-2032). This segment holds the dominant market share due to rising demand for testing supplies in hospitals, diagnostic centers, and increasingly at-home settings. After COVID-19 shift toward frequent respiratory testing growing the demand for combo kits that detect influenza alongside other viruses like SARS-CoV2 and RSV. Innovations in reagent stability and cold-chain independence are also making these products more accessible globally.
North America Influenza Diagnostic Market Regional Analysis:
The United States dominated the Market in 2024 and is expected to hold the largest Market share over the forecast period (2024-2032)
The dominance of United States in North America Influenza diagnostic market is primarily attributed to robust healthcare infrastructure and high diagnostic sensitivity. The United States anticipated high volume of annual tests because of government-initiated influenza surveillance schemes and regular outbreaks during winter months. The United States and Canada have well-established health care infrastructure with wide availability of hospitals, clinics and clinical laboratories. Robust infrastructure offers itself for the application of highly sensitive molecular assays such as RT-PCR.
North America Influenza Diagnostic Market Competitive Landscape
North America Influenza Diagnostics Market mainly dominated by some key players like Abbott Laboratories, Thermo Fisher Scientific, Quidel Corporation, Becton Dickinson. These companies hold more than 61% of the North America market share in 2024, due to their wide-ranging product portfolio, strong brand image and well -established distribution channels. These leading players invest heavy in research and development, and their main focus is on rapid and multiplex molecular clinical tests capable of detecting several respiratory viruses.
Recent development in North America Influenza Diagnostic Market
|
Date
|
Company |
Development |
|
April 2, 2024 |
Thermo Fisher Scientific |
Thermo Fisher announced a strategic expansion of its TaqPath Respiratory Viral Panel portfolio, including an upgraded multiplex RT-PCR assay that covers 20 respiratory targets, including flu strains. |
|
March 5, 2024 |
Becton Dickinson |
launched a new version of its Veritor Plus System with cloud-based connectivity for real-time reporting and tracking of influenza A/B, RSV, and COVID-19. |
|
February 14, 2024 |
QuidelOrtho Corporation |
QuidelOrtho announced the launch of an enhanced Sofia 2 Flu + COVID Antigen FIA in North America. |
|
January 8, 2024 |
Abbott Laboratories |
Abbott received FDA clearance for its Alinity m Resp-4-Plex assay, a multiplex molecular test that detects and differentiates between influenza A, influenza B, RSV, and COVID-19. |
|
North America Influenza Diagnostic Market Scope |
|
|
Market Size in 2024 |
USD 575 Bn. |
|
Market Size in 2032 |
USD 966.01 Bn. |
|
CAGR (2024-2032) |
6.7% |
|
Historic Data |
2019-2024 |
|
Base Year |
2024 |
|
Forecast Period |
2025-2032 |
|
Segments |
By Product Test kits and reagents Instruments Other Products |
|
By Test Type Molecular Diagnostic Tests Transcription-Mediated Amplification-Based Assay Traditional Diagnostic Tests Others |
|
|
By End-user Diagnostic Laboratories Hospitals and Clinics Others |
|
|
Regional Scope |
North America- United States, Canada, and Mexico |
Key Players in the Influenza Diagnostic Market
- Abbott Laboratories (United States)
- Thermo Fisher Scientific (United States)
- QuidelOrtho Corporation (United States)
- Becton, Dickinson and Company – BD (United States)
- Hologic Inc. (United States)
- Cepheid (United States)
- Meridian Bioscience (United States)
- BioFire Diagnostics (United States)
- GenMark Diagnostics (United States)
- Luminex Corporation (United States)
Frequently Asked Questions
The Market Faces Restraints Related to Limited Accessibility of Advanced Diagnostic Technologies.
Home-Based Influenza Testing and Digital Health Integration is an Opportunity for Influenza Diagnostic Market.
1. North America Influenza Diagnostic Market Introduction
1.1. Study Assumptions and Market Definition
1.2. Scope of the Study
1.3. Executive Summary
2. North America Influenza Diagnostic Market: Competitive Landscape
2.1. SMR Competition Matrix
2.2. Key Players Benchmarking
2.2.1. Company Name
2.2.2. Service Segment
2.2.3. End-User Segment
2.2.4. Revenue (2024)
2.2.5. Geographical Presence
2.3. Market Structure
2.3.1. Market Leaders
2.3.2. Market Followers
2.3.3. Emerging Players
2.4. Mergers and Acquisitions Details
3. North America Influenza Diagnostic Market: Dynamics
3.1. North America Influenza Diagnostic Market Trends
3.2. North America Influenza Diagnostic Market Dynamics
3.2.1. Drivers
3.2.2. Restraints
3.2.3. Opportunities
3.2.4. Challenges
3.3. PORTER’s Five Forces Analysis
3.4. PESTLE Analysis
3.5. Regulatory Landscape by Region
3.6. Key Opinion Leader Analysis for the Global Industry
3.7. Analysis of Government Schemes and Initiatives for Industry
4. North America Influenza Diagnostic Market: Market Size and Forecast by Segmentation (by Value in USD Mn) (2024-2032)
4.1. North America Influenza Diagnostic Market Size and Forecast, By Product (2024-2032)
4.1.1. Test kits and reagents
4.1.2. Instruments
4.1.3. Other Products
4.2. North America Influenza Diagnostic Market Size and Forecast, By Test Type (2024-2032)
4.2.1. Molecular Diagnostic Tests
4.2.2. Transcription-Mediated Amplification-Based Assay
4.2.3. Traditional Diagnostic Tests
4.2.4. Others
4.3. North America Influenza Diagnostic Market Size and Forecast, By End-user (2024-2032)
4.3.1. Diagnostic Laboratories
4.3.2. Hospitals and Clinics
4.3.3. Others
4.4. North America Influenza Diagnostic Market Size and Forecast, By Country (2024-2032)
4.4.1. United States
4.4.2. Canada
4.4.3. Mexico
5. Company Profile: Key Players
5.1 Abbott Laboratories
5.1.1. Company Overview
5.1.2. Business Portfolio
5.1.3. Financial Overview
5.1.4. SWOT Analysis
5.1.5. Strategic Analysis
5.1.6. Recent Developments
5.2 Abbott Laboratories
5.3 Thermo Fisher Scientific
5.4 QuidelOrtho Corporation
5.5 Becton, Dickinson and Company – BD
5.6 Hologic Inc.
5.7 Cepheid
5.8 Meridian Bioscience
5.9 BioFire Diagnostics
5.10 GenMark Diagnostics
5.11 Luminex Corporation
6 Key Findings
7 Industry Recommendations
















