France Pediatric Medical Device Market Size, Share, Growth Trends, Industry Analysis, Key Players, Investment Opportunities and Forecast (2025-2032)
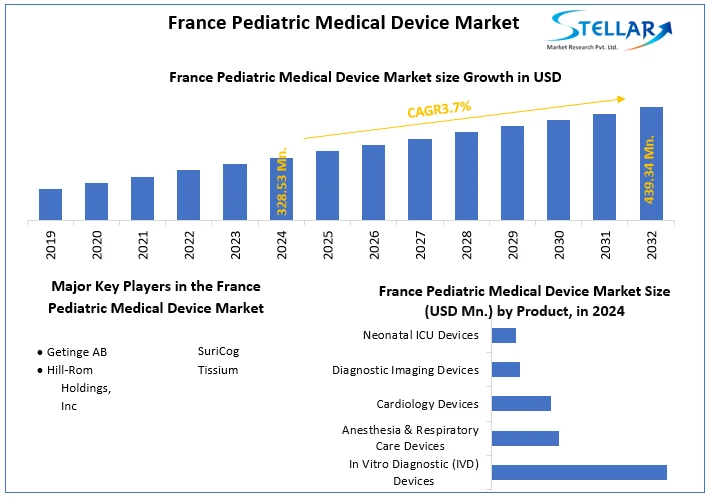
The France Pediatric Medical Device Market size was valued at USD 328.53 Mn in 2024 and the total France Pediatric Medical Device revenue is expected to grow at a CAGR of 3.7% from 2025 to 2032, reaching nearly USD 439.34 Mn in 2032.
Format : PDF | Report ID : SMR_1666
France Pediatric Medical Device Market Overview
Pediatric medical devices are employed to treat or diagnose diseases in children under the age of 21. Paediatrics is a branch of medical treatment for newborns, kids, and teenagers. Pediatric medical devices take into account factors such as smaller body sizes, developmental stages, and the unique physiology of pediatric patients.
The report analyzes several insights into the current market scenario, emerging trends, and possibilities. The report consists analysis of industry professionals, policymakers, and investors who stand to gain actionable intelligence through a strategic examination of market dynamics, technological strides, and key stakeholders. Focusing exclusively on pediatric medical devices, the report aims to empower decision-makers by presenting a holistic approach to the market landscape and its potential evolution. France's Pediatric Medical Device market stands out for its innovative approach, meeting unique healthcare needs for children with a diverse range of diagnostic, therapeutic, and monitoring solutions. The report navigates through the market's nuances, covering various specialties and addressing challenges while providing a detailed overview of France's pediatric medical devices.
The rise in the number of healthcare providers who prioritize patient-centric care approaches, leading to the development of pediatric medical devices tailored to the unique needs and preferences of children. Technological innovations have led to the development of minimally invasive techniques for pediatric surgeries and interventions.
- Out of the 1,300 enterprises in France, 92% are SMEs, of which 88% exclusively produce medical devices.
- The French market for medical prostheses accounts for approximately 7% of the total medical device market.
To get more Insights: Request Free Sample Report
Driving Factors for Growth in the Pediatric Medical Device Market in France
France is expected to witness an increase in Pediatric Medical device market growth owing to an increase in chronic childhood illnesses such as obesity, asthma, and diabetes. The increasing demand for specialized medical equipment to diagnose and manage the various diseases that occur in children has propelled the market growth. Ongoing government initiatives in France which influence the padiatric medical device manufacturers to invest in child healthcare have accelerated the market growth. As france plays a significant role in the strong foundation of the medical devices industry resulting in a greater demand for medical devices. The rapid development in technologies has contributed to well-developed pediatric medical devices.
Challenges and Limitations in the Pediatric Medical Device Market in France
The market for medical devices for children in France is kind of small compared to the market for grown-up medical devices. Many manufacturers decide not to focus on making new devices for kids because they might not earn as much money from them. As the market is small, there might also be fewer different choices of medical devices for children in France. Obtaining regulatory approval for pediatric devices involves additional testing and data specific to children's unique physiology, leading to increased time and cost compared to adult devices.
France Pediatric Medical Device Market Segment Analysis
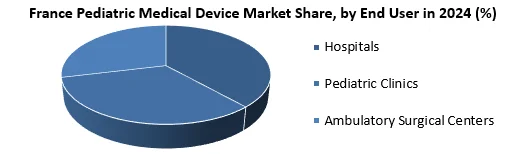
Based on End Users, the Hospitals segment holds the highest number of shares with a growing CAGR of 4.8% during the forecast period. Pediatric Medical devices play an important role in the delivery of many healthcare services to children. Hospitals perform a variety of pediatric medical procedures that require a variety of specialized equipment for diagnosis, treatment, and monitoring. Hospitals tend to handle large pediatric cases resulting in demand for pediatric medical devices.
The procurement process for hospitals is dynamic and involves numerous steps which include complex and evolving regulations that hospitals must follow. Depending on the technology, medical devices used to treat pediatric patients vary depending on whether it meet or exceed the demand for these technologies.
|
France Pediatric Medical Device Market Scope |
|
|
Market Size in 2024 |
USD 328.53 Million |
|
Market Size in 2032 |
USD 439.34 Million |
|
CAGR (2025-2032) |
3.7% |
|
Historic Data |
2019-2024 |
|
Base Year |
2024 |
|
Forecast Period |
2025-2032 |
|
Segment Scope |
By Product
|
|
By End User
|
|
France Pediatric Medical Device Market Key Players
- Getinge AB
- Hill-Rom Holdings, Inc
- SuriCog
- Tissium
Frequently Asked Questions
Stringent regulation and Rising costs are expected to be the major restraining factors for the France Pediatric Medical Device market growth.
The France Pediatric Medical Device Market size was valued at USD 328.53 Million in 2024 and the total France Pediatric Medical Device revenue is expected to grow at a CAGR of 3.7 % from 2025 to 2032, reaching nearly USD 439.34 Million By 2032.
1. France Pediatric Medical Device Market: Research Methodology
1.1. Research Data
1.1.1. Secondary Data
1.1.2. Primary Data
1.2. Market Size Estimation
1.2.1. Bottom-Up Approach
1.2.2. Top-Down Approach
1.3. Market Breakdown and Data Triangulation
1.4. Research Assumption
2. France Pediatric Medical Device Market: Executive Summary
2.1. Market Overview
2.2. Market Size (2024) and Forecast (2025 – 2032) and Y-O-Y%
2.3. Market Size (USD) and Market Share (%) – By Segments
3. France Pediatric Medical Device Market: Competitive Landscape
3.1. SMR Competition Matrix
3.2. Key Players Benchmarking
3.2.1. Company Name
3.2.2. Headquarter
3.2.3. Product Segment
3.2.4. End-user Segment
3.2.5. Y-O-Y%
3.2.6. Revenue (2024)
3.2.7. Company Locations
3.3. Market Structure
3.3.1. Market Leaders
3.3.2. Market Followers
3.3.3. Emerging Players
3.4. Consolidation of the Market
4. France Pediatric Medical Device Market: Dynamics
4.1. France Pediatric Medical Device Market Trends
4.2. France Pediatric Medical Device Market Drivers
4.3. France Pediatric Medical Device Market Restraints
4.4. France Pediatric Medical Device Market Opportunities
4.5. France Pediatric Medical Device Market Challenges
4.6. PORTER’s Five Forces Analysis
4.7. PESTLE Analysis
4.8. Value Chain Analysis
4.9. Technological Roadmap
4.10. Regulatory Landscape
5. France Pediatric Medical Device Market: Market Size and Forecast by Segmentation (by Value in USD Million) (2024-2032)
5.1. France Pediatric Medical Device Market Size and Forecast, by Product (2024-2032)
5.1.1. In Vitro Diagnostic (IVD) Devices
5.1.2. Anesthesia & Respiratory Care Devices
5.1.3. Cardiology Devices
5.1.4. Diagnostic Imaging Devices
5.1.5. Neonatal ICU Devices
5.2. France Pediatric Medical Device Market Size and Forecast, by End User (2024-2032)
5.2.1. Hospitals
5.2.2. Pediatric Clinics
5.2.3. Ambulatory Surgical Centers
6. Company Profile: Key Players
6.1. Getinge AB
6.1.1. Company Overview
6.1.2. Business Portfolio
6.1.3. Financial Overview
6.1.4. SWOT Analysis
6.1.5. Strategic Analysis
6.1.6. Recent Developments
6.2. Hill-Rom Holdings, Inc
6.3. SuriCog
6.4. Tissium
7. Key Findings
8. Analyst Recommendations
8.1. Strategic Recommendations
8.2. Future Outlook
















