Europe Pediatric Medical Device Market- Industry Analysis and Forecast (2025-2032)
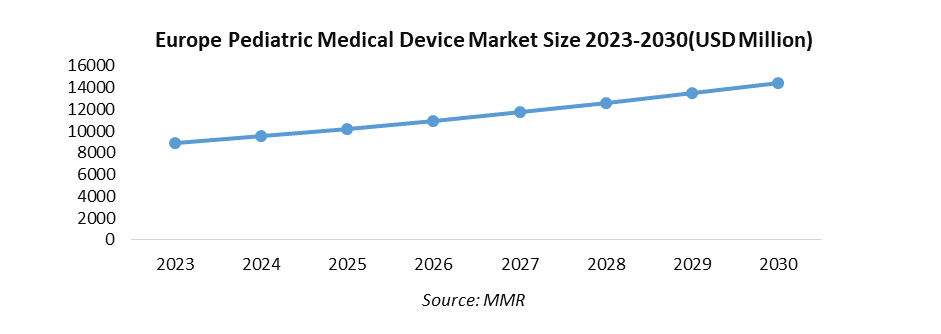
The Europe Pediatric Medical Device Market size was valued at USD 9537.80 Mn in 2024 and revenue is expected to grow at a CAGR of 7.13% from 2025 to 2032, reaching nearly USD 16547.69 Mn.
Format : PDF | Report ID : SMR_1664
Europe Pediatric Medical Device Market Overview
Pediatric medical devices are employed to treat or diagnose diseases in children under the age of 21. Paediatrics is a branch of medical treatment for newborns, kids, and teenagers. Pediatric medical devices take into account factors such as smaller body sizes, developmental stages, and the unique physiology of pediatric patients.
Paediatricians across Europe have voiced serious concerns about the evolving shortages of medical devices in the European Union (EU) that are essential for treating sick children. In 2024, the European Union revised its regulatory framework for medical devices, primarily to improve patient safety and public health. Germany dominated the European market with a major revenue share in 2024. As the medical device market for pediatrics grows, industry, academics, and clinicians have faced the formidable challenge of how to impact upon the hard-to-reach and vulnerable populations.
Addressing social determinants of child health and child health inequalities in large populations has been lighted by socioeconomic factors that limit technology reach. The substantial chronic disease burden, coupled with advanced healthcare infrastructure and easy access to services, continues to support the potential growth of the market. Additionally, caution is to be exercised as the Government, like many others in Europe, is controlling costs through various measures.
- In Europe, an average of approximately 11% of gross domestic product (GDP) is spent on healthcare. The spending on medical technology is estimated to vary significantly across European countries, ranging from around 5% to 12% of the total healthcare expenditure.

To get more Insights: Request Free Sample Report
Europe Pediatric Medical Device Market Dynamics
Driving Factors in the Growth of the European Pediatric Medical Devices Market
The rise in the incidences of chronic disease or infectious diseases among the pediatric population such as diphtheria, leukemia, measles, mumps, anemia, asthma, chickenpox, tuberculosis, whooping cough, Lyme disease, and pneumonia acts as one of the major factors driving the growth of pediatric medical devices market. The generic drug market is growing robustly, with the government's cost-containment policy and a significant number of patent expiries driving the growth of the market. The increase in demographic and epidemiological profiles provides growth opportunities for manufacturers of chronic treatments that have influenced the European pediatric medical devices market growth.
The increasing demand for pediatric medical equipment, primarily driven by the growing global pediatric population, is one of the key factors propelling the market. Additionally, the rising prevalence of pediatric chronic conditions such as diabetes, asthma, cancer, and heart disease is contributing to the heightened need for pediatric medical equipment and has boosted the market growth.
Challenges and Barriers Facing the European Pediatric Medical Devices Market
Paediatricians across Europe have voiced serious concerns about the evolving shortages of medical devices in the European Union (EU) that are essential for treating sick children. Pediatric medical devices are withdrawn from the market due to markedly increased regulatory requirements and high costs of certifying devices under the revised EU Medical Device Regulation. Despite numerous legislative, regulatory, and scientific efforts in recent years, innovative pediatric medical devices (PMDs) have yet to be made available at the same rate as adult medical devices. Many innovative medical devices approved for adult use have not been approved for pediatric use resulting in hindering the market growth.
Medical device development for pediatrics has remained relatively stagnant, as evidenced by premarket approvals (PMAs) and humanitarian device exemptions (HDEs) with labeling for pediatric usage. A major barrier to enabling the appropriate availability of medical devices for the pediatric age group is the very high financial cost of their assessment as a result it arises from the delegation of the conformity assessment in the EU to profit-making private enterprises that serve as notified bodies, with no prerequisites taken to encourage the certification of pediatric or orphan devices at low cost.
Europe Pediatric Medical Device Market Segment Analysis
Based on Product, In Vitro Diagnostic (IVD) Devices segment holds the highest share with a growing CAGR of 7.20% during the forecast period. In vitro diagnostics (IVDs) are non-invasive tests used on biological samples, for instance, blood, urine tissues, etc., to determine the status of a person’s health. Rising Awareness of preventative medicine and a robust regulatory framework that ensures the quality and efficacy of diagnostic tools have propelled the market growth. Medical technologies have improved the quality of care and the efficiency and sustainability of healthcare systems resulting in accelerating the market growth. There are over 40,000 different IVD products on the European market. The United Kingdom, Germany, France, Italy, and Spain play vital roles in the European in Vitro Diagnostic market.
Europe Pediatric Medical Device Market Regional Insights
Germany dominated the Pediatric Medical Device Market with the highest market share accounting for 33.80% in 2024, the Country is expected to grow during the forecast period and maintain its dominance by 2032. Thanks to the increasing adoption of advanced medical treatments. The rapidly increasing pediatric healthcare market, as well as the advances in digital healthcare and data analytics, has provided an opportunity to collect large volumes of meaningful national and international data to provide clarity about childhood growth, development, and disease. Additionally, a sizable patient base with low birth weight and an uptick in preterm deliveries are expected to drive market growth in Europe.
The German pediatric medical devices market was attributed to holding the largest market share, and the UK pediatric medical devices market is estimated to be the fastest growing market in the Europe region. Additionally, growing parental awareness programs and an increase in investment in research and development activities are driving the France pediatric medical devices market.
Europe Pediatric Medical Device Market Competitive Landscape
- In 2023, Medtronic PLC, the global leader in healthcare technology announced it filed a complaint with the U.S. International Trade Commission (ITC), along with a parallel action in the U.S. District Court for the District of Delaware, to block Axonics from improperly importing and selling products that infringe two Medtronic patents related to the MRI compatibility of implantable medical devices.
- In 2021, Stryker Corporation acquired Wright Medical Corporation for USD 5.4 Billion. Wright Medical has a strong presence in the pediatric orthopedics market and the acquisition increases Stryker's product portfolio in Europe.
|
Europe Pediatric Medical Device Market Scope |
|
|
Market Size in 2024 |
USD 9537.80 Mn. |
|
Market Size in 2032 |
USD 16547.69 Mn. |
|
CAGR (2025-2032) |
7.13% |
|
Historic Data |
2019-2024 |
|
Base Year |
2024 |
|
Forecast Period |
2025-2032 |
|
Segment Scope |
By Product
|
|
By End User
|
|
|
Country Scope |
|
Europe Pediatric Medical Device Key Players
- Medtronic PLC
- Hamilton Medical AG (Hamilton Bonaduz AG)
- TSE spol. s.r.o. (TSE Medical)
- Atom Medical Corporation
- F. Hoffmann-La Roche Ltd.
- Fritz Stephan GmbH
- General Electric Healthcare
- Hamilton Medical
- Koninklijke Philips N.V.
- Siemens AG
Country Breakdown:
France Pediatric Medical Device Market: Industry Analysis and Forecast (2024-2030)
Germany Pediatric Medical Device Market: Industry Analysis and Forecast (2024-2030)
Frequently Asked Questions
Supply Chain disruptions and Reimbursement uncertainties are expected to be the major restraining factors for the market growth.
China is expected to lead the global Europe Pediatric Medical Device market during the forecast period.
The Market size was valued at USD 9537.80 Million in 2024 and the total Market revenue is expected to grow at a CAGR of 7.13% from 2025 to 2032, reaching nearly USD 16547.69 Million.
The segments covered in the market report are By Product and End User.
1. Europe Pediatric Medical Device Market: Research Methodology
1.1. Research Data
1.1.1. Secondary Data
1.1.2. Primary Data
1.2. Market Size Estimation
1.2.1. Bottom-Up Approach
1.2.2. Top-Down Approach
1.3. Market Breakdown and Data Triangulation
1.4. Research Assumption
2. Europe Pediatric Medical Device Market: Executive Summary
2.1. Market Overview
2.2. Market Size (2024) and Forecast (2025 – 2032) and Y-O-Y%
2.3. Market Size (USD) and Market Share (%) – By Segments
3. Europe Pediatric Medical Device Market: Competitive Landscape
3.1. SMR Competition Matrix
3.2. Key Players Benchmarking
3.2.1. Company Name
3.2.2. Headquarter
3.2.3. Product Segment
3.2.4. End-user Segment
3.2.5. Y-O-Y%
3.2.6. Revenue (2024)
3.2.7. Profit Margin
3.2.8. Market Share
3.2.9. Company Locations
3.3. Market Structure
3.3.1. Market Leaders
3.3.2. Market Followers
3.3.3. Emerging Players
3.4. Consolidation of the Market
4. Europe Pediatric Medical Device Market: Dynamics
4.1. Europe Pediatric Medical Device Market Trends
4.2. Europe Pediatric Medical Device Market Drivers
4.3. Europe Pediatric Medical Device Market Restraints
4.4. Europe Pediatric Medical Device Market Opportunities
4.5. Europe Pediatric Medical Device Market Challenges
4.6. PORTER’s Five Forces Analysis
4.7. PESTLE Analysis
4.8. Value Chain Analysis
4.9. Technological Roadmap
4.10. Regulatory Landscape
5. Europe Pediatric Medical Device Market: Market Size and Forecast by Segmentation (by Value in USD Million) (2024-2032
5.1. Europe Pediatric Medical Device Market Size and Forecast, by Product (2024-2032
5.1.1. In Vitro Diagnostic (IVD) Devices
5.1.2. Anesthesia & Respiratory Care Devices
5.1.3. Cardiology Devices
5.1.4. Diagnostic Imaging Devices
5.1.5. Neonatal ICU Devices
5.2. Europe Pediatric Medical Device Market Size and Forecast, by End User (2024-2032
5.2.1. Hospitals
5.2.2. Pediatric Clinics
5.2.3. Ambulatory Surgical Centers
5.3. Europe Pediatric Medical Device Market Size and Forecast, by Country (2024-2032
5.3.1. Germany
5.3.2. United Kingdom
5.3.3. Spain
5.3.4. France
5.3.5. Italy
5.3.6. Belgium
5.3.7. Sweden
5.3.8. Poland
5.3.9. Russia
6. Company Profile: Key Players
6.1. Medtronic PLC
6.1.1. Company Overview
6.1.2. Business Portfolio
6.1.3. Financial Overview
6.1.4. SWOT Analysis
6.1.5. Strategic Analysis
6.1.6. Recent Developments
6.2. Hamilton Medical AG (Hamilton Bonaduz AG)
6.3. TSE spol. s.r.o. (TSE Medical)
6.4. Atom Medical Corporation
6.5. F. Hoffmann-La Roche Ltd.
6.6. Fritz Stephan GmbH
6.7. General Electric Healthcare
6.8. Hamilton Medical
6.9. Koninklijke Philips N.V.
6.10. Siemens AG
7. Key Findings
8. Analyst Recommendations
8.1. Strategic Recommendations
8.2. Future Outlook
















