US Pediatric Medical Device Market Size, Share, Growth Trends, Industry Analysis, Key Players, Investment Opportunities and Forecast (2025-2032)
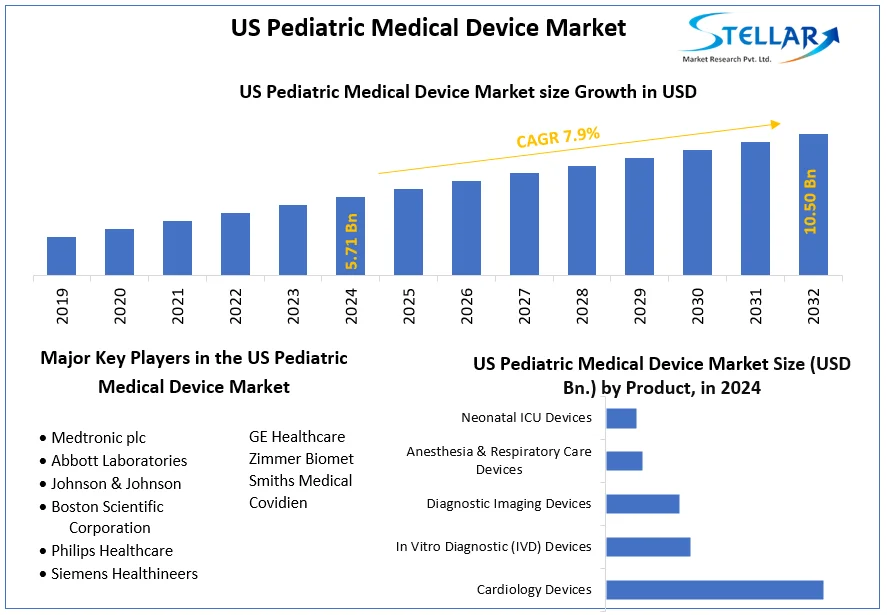
The US Pediatric Medical Device Market size was valued at USD 5.71 Billion in 2024 and the total US Pediatric Medical Device revenue is expected to grow at a CAGR of 7.9 % from 2025 to 2032, reaching nearly USD 10.50 Billion.
Format : PDF | Report ID : SMR_1603
US Pediatric Medical Device Market Overview
Pediatric medical devices address conditions from birth to age 21 as per the Categories including neonates, infants, children, and adolescents. The devices are designed for children or adapted from adult ones and pose challenges due to size, activity, and evolving physiology. Long-term use raises concerns about device durability and exposure to implanted materials.
|
This report analyses the US Pediatric Medical Device market and covers trends, technological advancements, and potential disruptions that shape the market. It assesses market size, growth, economic factors, regulations, and commercial drivers. The competitive landscape is analyzed, highlighting differentiation among key operators and drawing on historical data, industry insights, and the report forecasts sector. The economic downturn prompted this analysis and revealed the US Pediatric Medical Device industry's resilience challenges. The report aims to equip stakeholders with crucial, concise information for informed decision-making in this dynamic sector. The targeted audiences include People with Healthcare professionals, Secondary Audiences, Government agencies, policymakers, and pharmaceutical companies in the US Pediatric Medical Device industry.
To get more Insights: Request Free Sample Report
Growing Demand for Specialized Care Impact on the US Pediatric Medical Device Market
In the US Pediatric Medical Device Market Growing importance of specialized treatments drives innovation in pediatric care, prompting increased research investment. New devices improve minimally invasive procedures, remote monitoring, and personalized medicine, potentially improving outcomes for young patients.
The US Pediatric Medical Device Market navigating regulatory hurdles due to stringent safety and efficacy criteria, delaying market entry for new technologies. Smaller production volumes and higher per-unit costs strain healthcare budgets, potentially limiting patient access. Reimbursement policies lagging behind technological advancements pose challenges for hospitals and clinics in adopting the latest innovations.
Impact of Government Initiatives and Policies on the US Pediatric Medical Device Market
|
Initiative/Policy |
Positive Impact |
Negative Impact |
|
Funding for Pediatric Device Research and Development |
Stimulates the development of new and innovative devices tailored to children's unique needs. -Increases availability of diverse products for various pediatric conditions. -Fosters collaboration between academia, industry, and healthcare providers. |
Limited funding does not address all unmet needs or support smaller, early-stage companies. -Complex application and approval processes hinder participation. |
|
Regulatory Streamlining for Pediatric Devices |
Expedites regulatory approval for low-risk devices with potential benefit to children. - Reduces development costs and time to market for manufacturers. - Encourages investment in pediatric-specific devices. |
Potential safety concerns if streamlined pathways compromise thorough evaluation. -Suitable for all device types or with significant risk profiles. |
|
Coverage and Reimbursement Incentives |
Increases access to necessary devices for children with diverse insurance coverage. - Promotes adoption of new technologies with demonstrably improved outcomes. - Creates a financially viable market for pediatric device manufacturers. |
Complex and inconsistent reimbursement policies create barriers for some devices and limit access. - Bureaucratic hurdles and documentation requirements add burden to providers. - Limited coverage for newer or specialized devices persists. |
|
Tax Credits and Incentives for Pediatric Device Development |
Provides financial support for manufacturers to invest in R&D and innovation. - Attracts new entrants and encourages continued development in the Pediatric Medical Device market. -lower device costs and improve affordability for patients. |
Limited scope or funding availability, benefiting only a select few companies. - Effectiveness in driving significant market changes requires careful design and evaluation. |
|
Public Awareness Campaigns and Education Initiatives |
Raises awareness about the importance of Pediatric -specific devices and their benefits. -Educates healthcare providers, parents, and policymakers about available options. -Creates a more informed and receptive market for new technologies. |
Requires sustained funding and effective communication strategies to reach target audiences. -Overcome ingrained biases or preferences for established adult devices. |
|
Focus on Telehealth and Remote Monitoring Technologies |
Enlarges access to care for children in remote areas or with mobility limitations. - Improves convenience and efficiency of care delivery for families. -Creates opportunities for new and innovative remote monitoring devices. |
Requires investments in digital infrastructure and addressing connectivity disparities. - Cybersecurity and data privacy concerns need proactive measures and clear regulations. -Integration with existing healthcare IT systems needs standardization and improvement. |
US Pediatric Medical Device Market Segment Analysis
Based on End-users, the Hospitals segment held the largest market share of about 40% in the US Pediatric Medical Device Market in 2023. According to the STELLAR analysis, the segment is further expected to grow at a CAGR of 8.1% during the forecast period. It stands out as the dominant segment within the US Pediatric Medical Device Market thanks to its rapid technological advancement and growing adoption of smart devices with data connectivity and integration.
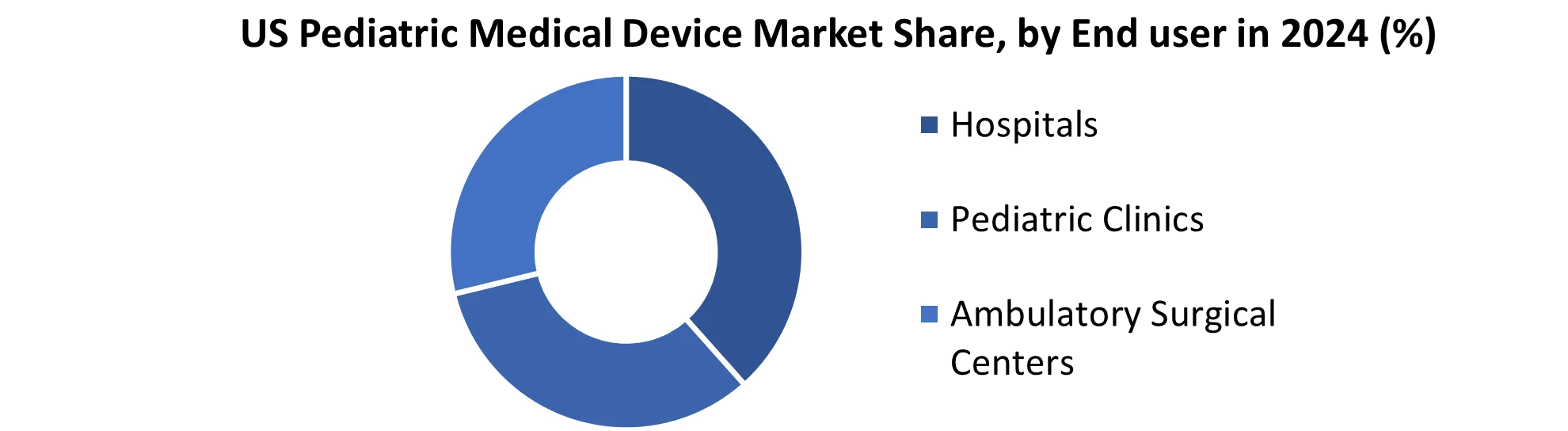
Hospitals, command the largest US Pediatric Medical Device Market share and drive the demand for diverse medical devices, embracing innovations such as robotic surgical systems and AI diagnostics. Collaborations drive clinical R&D, expediting regulatory approvals. Pediatric clinics witness growth, emphasizing outpatient and preventive care, leading to increased adoption of smaller, portable devices. Telehealth and remote monitoring gain traction, enhancing chronic disease management and remote consultations. Home care sees rising demand for user-friendly devices, enabling continuous monitoring and improving patient satisfaction by providing care in familiar surroundings, thus enhancing comfort and recovery.
Limited reimbursement, especially for newer technologies, hampers device adoption across end-user segments. Stringent documentation burdens providers, discouraging device use. Lack of standardization in data formats and communication protocols poses integration challenges, hindering interoperability. Privacy and security concerns in remote monitoring necessitate robust cybersecurity, while ethical data collection practices are vital for widespread adoption.
The US Pediatric Medical Device Market booms on the increasing need for accessible, convenient, and personalized care, driven by technological advancements. To ensure sustainable growth and equitable access, addressing reimbursement, standardization, and privacy challenges is crucial. Incorporate data, discuss emerging trends like telehealth and AI, and analyze the competitive landscape for a comprehensive understanding.
US Pediatric Medical Device Market Scope:
|
US Pediatric Medical Device Market Scope |
|
|
Market Size in 2024 |
USD 5.71 Billion |
|
Market Size in 2032 |
USD 10.50 Billion |
|
CAGR (2025-2032) |
7.9 % |
|
Historic Data |
2019-2024 |
|
Base Year |
2024 |
|
Forecast Period |
2025-2032 |
|
Segment Scope |
By Product
|
|
By End-user
|
|
Leading Key Players in the US Pediatric Medical Device Market
- Medtronic plc
- Abbott Laboratories
- Johnson & Johnson
- Boston Scientific Corporation
- Philips Healthcare
- Siemens Healthineers
- GE Healthcare
- Zimmer Biomet
- Smiths Medical
- Covidien
Frequently Asked Questions
Increased awareness and increasing prices are expected to be the major restraining factors for the US Pediatric Medical Device market growth.
The US Pediatric Medical Device Market size was valued at USD 5.71 Billion in 2024 and the total US Pediatric Medical Device revenue is expected to grow at a CAGR of 7.9 % from 2025 to 2032, reaching nearly USD 10.50 Billion By 2032.
1. US Pediatric Medical Device Market Introduction
1.1 Study Assumption and Market Definition
1.2 Scope of the Study
1.3 Executive Summary
1.4 Emerging Technologies
1.5 Market Projections
1.6 Strategic Recommendations
2. Impact of Telehealth and Remote Monitoring on the US Pediatric Medical Device Market
2.1 Increased Access to Care
2.2 Enhanced Early Detection
2.3 Empowered Caregiver
3. US Pediatric Medical Device Market: Dynamics
3.1.1 Market Drivers
3.1.2 Market Restraints
3.1.3 Market Opportunities
3.1.4 Market Challenges
3.2 PORTER’s Five Forces Analysis
3.3 PESTLE Analysis
3.4 Regulatory Landscape
3.5 Analysis of Government Schemes and Initiatives for the US Pediatric Medical Device Industry
3.6 The Pandemic and Redefining of The US Pediatric Medical Device Industry Landscape
4. US Pediatric Medical Device Market: Market Size and Forecast by Segmentation (Value) (2024-2032)
4.1 US Pediatric Medical Device Market Size and Forecast, by Product (2024-2032)
4.1.1 Cardiology Devices
4.1.2 In Vitro Diagnostic (IVD) Devices
4.1.3 Diagnostic Imaging Devices
4.1.4 Anesthesia & Respiratory Care Devices
4.1.5 Neonatal ICU Devices
4.2 US Pediatric Medical Device Market Size and Forecast, by End-user (2024-2032)
4.2.1 Hospitals
4.2.2 Pediatric Clinics
4.2.3 Ambulatory Surgical Centers
5. US Pediatric Medical Device Market: Competitive Landscape
5.1 STELLAR Competition Matrix
5.2 Competitive Landscape
5.3 Key Players Benchmarking
5.3.1 Company Name
5.3.2 Service Segment
5.3.3 End-user Segment
5.3.4 Revenue (2024)
5.3.5 Company Locations
5.4 Leading US Pediatric Medical Device Companies, by market capitalization
5.5 Market Structure
5.5.1 Market Leaders
5.5.2 Market Followers
5.5.3 Emerging Players
5.6 Mergers and Acquisitions Details
6. Company Profile: Key Players
6.1 Medtronic plc
6.1.1 Company Overview
6.1.2 Business Portfolio
6.1.3 Financial Overview
6.1.4 SWOT Analysis
6.1.5 Strategic Analysis
6.1.6 Scale of Operation (small, medium, and large)
6.1.7 Details on Partnership
6.1.8 Regulatory Accreditations and Certifications Received by Them
6.1.9 Awards Received by the Firm
6.1.10 Recent Developments
6.2 Abbott Laboratories
6.3 Johnson & Johnson
6.4 Boston Scientific Corporation
6.5 Philips Healthcare
6.6 Siemens Healthineers
6.7 GE Healthcare
6.8 Zimmer Biomet
6.9 Smiths Medical
6.10 Covidien
7. Key Findings
8. Industry Recommendations
9. Terms and Glossary
10. US Pediatric Medical Device Market: Research Methodology
















