Asia Pacific Prefilled Syringes Market Size, Share, Growth Trends, Industry Analysis, Key Players, Investment Opportunities, and Forecast (2025-2032)
The Asia Pacific Prefilled Syringes Market size was valued at USD 1.33 Bn. in 2024 and the total Asia Pacific Prefilled Syringes Market revenue is expected to grow at a CAGR of 11% from 2025 to 2032, reaching nearly USD 3.06 Bn. in 2032.
Format : PDF | Report ID : SMR_1779
Asia Pacific Prefilled Syringes Market Overview
A pre-filled syringe is paired with the injectable substance and affixed needle by the manufacturer and offers a single-dose packet for vaccines and drugs. It provides cost-effectiveness, safety, convenience, reliability, sterility, and affordability and making it a preferred option for administering injectable medications and diluents. The Asia Pacific Prefilled Syringes market is surging thanks to favorable demographics such as a large population and increasing chronic diseases. China dominates the market for developed prefilled syringes, while Japan and India offer substantial growth prospects. Also rising foreign direct investment (FDI) and demand for products entice key firms into these Asia Pacific Prefilled Syringes markets.
- In April 2023, the FDA approved a new 50mL/10gm prefilled syringe for Hizentra® (immune globulin subcutaneous [human] 20% liquid).
The Asia Pacific Prefilled Syringes market report provides comprehensive insights into trends, tech innovations, and disruptions, assessing dynamics, size, regulations, and drivers. Benefiting healthcare, government, and pharma sectors, it facilitates informed decision-making amid economic hurdles, emphasizing competitive advantage through essential data.

To get more Insights: Request Free Sample Report
Asia Pacific Prefilled Syringes Market Dynamics
Asia Pacific Prefilled Syringes Market Driver:
In the Asia Pacific Prefilled Syringes Market, the increasing incidence of chronicle diseases like diabetes, cancer, and autoimmune disorders is remarkable. Also the factors such as population aging, urbanization, and lifestyle shifts contribute to this rise and given the need for long-term treatment involving injectable medications so prefilled syringes emerge as a convenient and safe option for administering these drugs and addressing the growing demand for effective therapeutic solutions amidst the region's changing healthcare landscape.
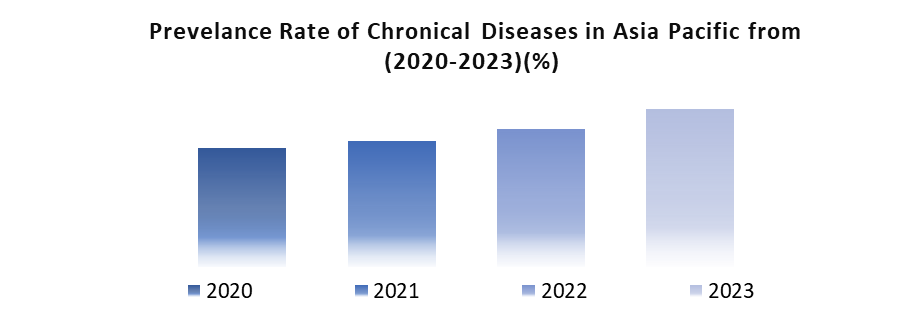
The increasing geriatric population, attributed to declining birth rates and extended life expectancy and presents a significant trend with older individuals prone to chronic illnesses necessitating injectable medications and prefilled syringes emerge as an optimal solution. Their user-friendly design and minimal training requirements cater well to this demographic, addressing the evolving healthcare needs of an aging population across the Asia Pacific region. In the Asia Pacific Prefilled Syringes Market, the uptake of self-injection therapies for chronic diseases is on the rise and fueled by factors like expanded access to self-injectable devices and escalating healthcare expenses. Prefilled syringes offer patients a safe and convenient means to self-administer medications and aligning with the growing preference for self-care solutions amidst the region's evolving healthcare landscape.
Asia Pacific Prefilled Syringes Market Challenges:
The Asia Pacific prefilled syringes market expected the highest CAGR globally. Also, show challenges, particularly the high costs compared to traditional syringes, hindering adoption in cost-sensitive markets such as India and Southeast Asia. Addressing these cost barriers is crucial for broader market penetration and sustained growth. In the Asia Pacific Prefilled Syringes Market, lack of awareness among healthcare professionals and patients about their benefits inhibits growth. Stringent regulations add complexity and deterring manufacturers. Additionally, competition from alternative drug delivery methods like auto injectors and pen injectors presents challenges and as they offer convenience and preference for specific medications.
- The stringent regulatory requirements pose significant challenges for manufacturers introducing new products. Navigating complex regulations in some countries lead to delays in market entry and impeding growth. These hurdles not only increase the time and resources required for approval but also create barriers for innovation and growth within the region. Regulatory harmonization efforts and streamlined approval processes are essential to foster a conducive environment for market development and ensure timely access to safe and effective prefilled syringe products.
- In the Asia Pacific Prefilled Syringes Market, inadequate cold chain infrastructure in certain regions poses challenges for storing and transporting temperature-sensitive products. This limitation particularly affects rural and remote areas where proper storage conditions are essential but lacking without robust cold chain facilities and the viability of prefilled syringes in reaching underserved populations diminishes, hindering their accessibility and adoption. Addressing this infrastructure gap through investment and strategic planning is imperative to ensure equitable distribution of prefilled syringe products and improve healthcare outcomes across the Asia Pacific region.
- The needle stick injuries pose a significant risk to healthcare workers. Safety features in prefilled syringes mitigate this risk and yet their higher cost compared to traditional syringes hinder widespread adoption. Balancing safety concerns with affordability is crucial to ensure broader utilization of prefilled syringes, addressing occupational hazards while maintaining cost-effectiveness in healthcare delivery across diverse markets in the Asia Pacific region.
Asia Pacific Prefilled Syringes Market Segment Analysis:
By Material, the Glass Based segment held the largest market share of about 60% in the Asia Pacific Prefilled Syringes Market in 2024. According to the STELLAR analysis, the segment is further expected to grow at a CAGR of 11.1% during the forecast period. The Asia Pacific Prefilled Syringes Market leads thanks to rapid tech advancement and adoption of smart devices with data connectivity and integration.

Glass-based prefilled syringes lead the Asia Pacific market thanks to their superior barrier properties and ensuring drug stability by preventing interactions with oxygen or water vapor. As the historically dominant player glass syringes offer unmatched chemical stability, crucial for maintaining drug integrity. This resilience positions them as the preferred choice in the region's prefilled syringes market segment. Glass offers broad compatibility with various drug formulations compared to some plastics. Also, the region boasts an established manufacturing infrastructure for glass prefilled syringes and further solidifying their position as a preferred choice in the market segment.
In the Asia Pacific Prefilled Syringes industry, glass syringes dominate due to their ability to ensure high drug stability, vital for sensitive medications needing protection from environmental factors with minimal risk of chemical reactions between drug and container, they offer low drug interaction, enhancing safety. Glass syringes also boast heat resistance, making them suitable for medications requiring heat sterilization. Additionally, their transparency allows visual inspection for clarity and presence of particles and ensuring quality control and further cementing their position as the preferred choice in the region.
The glass syringes hold prominence for high-value medications and biologics and benefiting from superior stability and barrier properties. However, they face challenges from plastic alternatives. Plastic advancements address compatibility and barrier limitations and broadening their applicability. Cost-effectiveness makes plastic syringes competitive, especially for large-volume production. While glass maintains significance and its dominance wane as plastic technology advances and cost considerations grow. However, for high-value and sensitive medications, the benefits of glass still outweigh the drawbacks, ensuring its continued presence in the Asia Pacific Prefilled Syringes market.
Asia Pacific Prefilled Syringes Market Regional Insights:
The Asia Pacific prefilled syringes market is poised as the fastest-growing globally and fueled by region-specific factors.
- China boasting a vast and aging population prone to chronic ailments demanding injectable treatments and drives the Asia Pacific prefilled syringes market. Also supported by escalating healthcare spending and governmental pushes for local medical device manufacturing and the nation witnesses a surge in biologics and self-administered injectable drugs adoption.
- India emerges as a promising frontier in the Asia Pacific prefilled syringes market and driven by surging rates of chronic conditions such as diabetes and autoimmune disorders with improving healthcare accessibility and rising disposable incomes. The nation witnesses governmental endeavors to enhance medical device affordability and availability and fostering market growth.
- South Korea emerges as a significant contender in the Asia Pacific prefilled syringes arena and driven by a robust healthcare infrastructure and burgeoning investments in biopharmaceutical research. Government backing for local manufacturers and the uptake of advanced drug delivery systems further fuels the market. Additionally, the aging populace emphasizes the need for medication adherence, stimulating growth.
- Japan constitutes a mature market for prefilled syringes and characterized by a high incidence of chronic ailments and advanced medical facilities. Stringent regulatory measures uphold product quality and safety standards. The escalating demand for prefilled syringes underscores the country's significance in the Asia Pacific region.
- Australia showcases a stable market for prefilled syringes and driven by governmental initiatives to enhance medication safety and mitigate errors. The nation embraces innovative technologies in prefilled syringes and witnessing high adoption rates. Also, there's a rising demand for home-based healthcare solutions, further fueling the market's growth trajectory.
- Malaysia, Indonesia, Vietnam, and Bangladesh represent increasing markets for prefilled syringes in Southeast Asia. Government investments in healthcare infrastructure and coupled with rising awareness of the benefits of prefilled syringes and drive market growth. As patient populations grow and access to essential medical technologies improves, demand escalates. These factors alongside others in the Asia Pacific region and present substantial growth opportunities for the prefilled syringes market, underpinned by favorable demographics, economic dynamics, and technological advancements.
Competitive Landscape for the Asia Pacific Prefilled Syringes Market
In the cutthroat Asia Pacific Prefilled Syringes (ADC) market, major contenders vie fiercely. Detailed profiles probe into company histories, finances, R&D endeavors, and market prospects, dissecting strengths, weaknesses, and product portfolios. With a regional focus, the landscape covers patent Intel, tech progressions, application trends, and production setups. Firms prioritize innovating cost-efficient solutions to drive market growth and employing strategic moves like tech partnerships and mergers to stay competitive. Collaborations and mergers serve as crucial tactics for firms navigating this ever-changing landscape, ensuring their continued relevance and dominance.
- The Asia-Pacific prefilled syringes market report delves into key players such as, Intas Pharmaceuticals Ltd., Health Biotech Limited, NIPRO, Daetwyler Holding, Bayer AG, SCHOTT AG, Gerresheimer AG, Haselmeier, Weigao Group, Catalent, Inc., Baxter, Medtronic, West Pharmaceutical Services, Inc., and Terumo Corporation. DBMR analysts offer insightful competitive analyses, dissecting strengths and strategies of each competitor individually and enhancing market comprehension and strategic decision-making.
In the Asia Pacific Prefilled Syringes market, strategic initiatives focus on diversification, segment-specific innovation, and partnership cultivation to improve competitiveness. These efforts adapt to changing consumer demands and positioning manufacturers for sustained growth and market prominence in the region's Prefilled Syringes sector.
|
Asia Pacific Prefilled Syringes Market Scope |
|
|
Market Size in 2024 |
USD 1.33 Billion |
|
Market Size in 2032 |
USD 3.06 Billion |
|
CAGR (2025-2032) |
11 % |
|
Historic Data |
2019-2024 |
|
Base Year |
2024 |
|
Forecast Period |
2025-2032 |
|
By Material
|
|
|
By Design
|
|
|
By Therapeutic
|
|
|
By Application
|
|
|
By Country
|
|
Asia Pacific Prefilled Syringes Market Key Players
- Abbott Laboratories
- Bayer AG
- Baxter International, Inc.
- Becton, Dickinson and Company
- Gerresheimer AG
- Medtronic PLC
- Nipro Corporation
- Terumo Corporation
- Vetter Pharma
- West Pharmaceutical Services, Inc.
- Stevanato Group
- WIPO
- Intas Phar
Country Breakdown:
Malaysia Prefilled Syringes Market: Industry Analysis and Forecast (2024-2030)
Indonesia Prefilled Syringes Market: Industry Analysis and Forecast (2024-2030)
Frequently Asked Questions
High Costs and the stringent regulatory requirements are expected to be the major restraining factors for the market growth.
China is expected to lead the Asia Pacific Prefilled Syringes market during the forecast period.
The Market size was valued at USD 1.33 Billion in 2024 and the total Market revenue is expected to grow at a CAGR of 11% from 2025 to 2032, reaching nearly USD 3.06 Billion.
The segments covered in the market report are By Material, Design, Therapeutic, and Application.
1. Asia Pacific Prefilled Syringes Market: Research Methodology
1.1. Research Data
1.1.1. Secondary Data
1.1.2. Primary Data
1.2. Market Size Estimation
1.2.1. Bottom-Up Approach
1.2.2. Top-Down Approach
1.3. Market Breakdown and Data Triangulation
1.4. Research Assumption
2. Asia Pacific Prefilled Syringes Market: Executive Summary
2.1. Market Overview
2.2. Market Size (2024) and Forecast (2025 – 2032) and Y-O-Y%
2.3. Market Size (USD) and Market Share (%) – By Segments
3. Asia Pacific Prefilled Syringes Market: Competitive Landscape
3.1. SMR Competition Matrix
3.2. Key Players Benchmarking
3.2.1. Company Name
3.2.2. Headquarter
3.2.3. Product Segment
3.2.4. End-user Segment
3.2.5. Y-O-Y%
3.2.6. Revenue (2024)
3.2.7. Profit Margin
3.2.8. Market Share
3.2.9. Company Locations
3.3. Market Structure
3.3.1. Market Leaders
3.3.2. Market Followers
3.3.3. Emerging Players
3.4. Consolidation of the Market
4. Asia Pacific Prefilled Syringes Market: Dynamics
4.1. Asia Pacific Prefilled Syringes Market Trends
4.2. Asia Pacific Prefilled Syringes Market Drivers
4.3. Asia Pacific Prefilled Syringes Market Restraints
4.4. Asia Pacific Prefilled Syringes Market Opportunities
4.5. Asia Pacific Prefilled Syringes Market Challenges
4.6. PORTER’s Five Forces Analysis
4.7. PESTLE Analysis
4.8. Technological Roadmap
4.9. Value Chain Analysis
4.10. Regulatory Landscape
5. Asia Pacific Prefilled Syringes Market: Market Size and Forecast by Segmentation (by Value in USD Million) (2024-2032)
5.1. Asia Pacific Prefilled Syringes Market Size and Forecast, by Material (2024-2032)
5.1.1. Glass-based
5.1.2. Plastic-based
5.2. Asia Pacific Prefilled Syringes Market Size and Forecast, by Design (2024-2032)
5.2.1. Single-chamber Prefilled Syringes
5.2.2. Dual-chamber Prefilled Syringes
5.2.3. Customized Prefilled Syringes
5.3. Asia Pacific Prefilled Syringes Market Size and Forecast, by Therapeutic (2024-2032)
5.3.1. Large Molecules
5.3.2. Small Molecules
5.4. Asia Pacific Prefilled Syringes Market Size and Forecast, by Application (2024-2032)
5.4.1. Anaphylaxis
5.4.2. Rheumatoid Arthritis
5.4.3. Diabetes
5.5. Asia Pacific Prefilled Syringes Market Size and Forecast, by Country (2024-2032)
5.5.1. China
5.5.2. India
5.5.3. South Korea
5.5.4. Japan
5.5.5. Australia
5.5.6. Malaysia
5.5.7. Indonesia
5.5.8. Vietnam
5.5.9. Bangladesh
6. Company Profile: Key Players
6.1. Abbott Laboratories
6.1.1. Company Overview
6.1.2. Business Portfolio
6.1.3. Financial Overview
6.1.4. SWOT Analysis
6.1.5. Strategic Analysis
6.1.6. Recent Developments
6.2. Bayer AG
6.3. Baxter International, Inc.
6.4. Becton, Dickinson and Company
6.5. Gerresheimer AG
6.6. Medtronic PLC
6.7. Nipro Corporation
6.8. Terumo Corporation
6.9. Vetter Pharma
6.10. West Pharmaceutical Services, Inc.
6.11. Stevanato Group
6.12. WIPO
6.13. Intas Phar
7. Key Findings
8. Analyst Recommendations
8.1. Strategic Recommendations
8.2. Future Outlook
















